One last, minor thing: Vanilla is a deeply rich flavor that has unfairly become shorthand for boring, basic, and sexually unadventurous. Merriam-Webster’s second definition includes the sad phrase “lacking distinction” to explain the term “vanilla.” I’m not arguing that we drop this secondary use of the word — we’re too far gone for that — but I do want to remind people that vanilla is actually an extraordinarily complex flavor. Chocolate is far more vanilla than vanilla.
Caitlin PenzeyMoog, “Salt grinders are bullshit, and other lessons from growing up in the spice trade”, The A.V. Club, 2017-04-06.
April 22, 2017
QotD: Vanilla isn’t
March 15, 2017
Using the Banana Equivalent Dose (BED) to measure hysteria in media reports on radiation
It’s quite common to find media reports involving radiation that are heavy on the freak-out factor and light on the facts. Here’s an interesting and useful rule of thumb you can use … in the few cases that the reports actually provide any meaningful figures on radioactivity:
Long-time readers know that very useful measures of both radioactivity and radiation dose rates are the Banana Equivalent Dose (BED), and a similar measure I think I invented (because no one else ever bothered) called the Banana Equivalent Radioactivity (BER). (The units here are explained in my old article “Understanding Radiation.”)
Bananas are useful for these measures because bananas concentrate potassium, and a certain amount of that potassium is ⁴⁰K, which is naturally radioactive. The superscript “40” there is the atomic number, or the number of protons in the nucleus, of that particular potassium (symbol K) isotope. Because of that potassium content, bananas are mildly radioactive: a medium banana at around 150g emits about 1 micro-Sievert per hour (1 µSv/hr) and contains about 15 Becquerel (15 Bq) of radioactive material.
(Why bananas? There are a lot of plant-based foods that concentrate potassium. It is, however, an essential rule of humor that bananas are the funniest fruit.)
Our radioactive boars are considered unfit at 600 Bq per kilogram. So, a tiny bit of arithmetic [(1000 g/kg)/150 g/banana × 15 Bq/banana] gives us 100 Bq/kg for bananas. All right, so this boar meat has 6 times as much radioactivity as a banana. Personally, this wouldn’t worry me.
So let’s turn to the radioactivity detected off the Oregon coast. This is 0.3 Bq per cubic meter. Conveniently — the joys of metric — one cubic meter of water is one metric tonne is 1000 liters is 1000 kilograms, so the radiation content here is .0003 Bq/kg.
15/0.0003 is 50,000. So, bananas have 50,000 times more radiation than the seawater being reported.
October 31, 2015
The chemistry of cider
At Compound Interest, a look at the making of cider:
On a hot summer’s day, the cool, refreshing taste of cider is hard to beat. But what are the chemicals behind this flavour?
Before we look at the chemistry, let’s briefly discuss how cider is made. Obviously, it starts with the apples being picked from the tree. The type of apples is, of course, a major factor in the taste of the finished cider. Bittersweet cider apples are low on acidity, but high on tannins, whilst sharp apples are the opposite. Sweet apples, meanwhile, are low in both departments, whilst bittersharp apples are high in both.
Once the apples have been picked, they’re left to mature for a time before then being scratted, or ground down, into a pulp. The pulp produced by this process is known as pomace. This pomace is then pressed to squeeze out all of the juice, which is collected into either vats or casks. At this point, it is then slowly fermented, and yeasts convert the natural sugars in the apples into alcohol. These yeasts can be the natural yeasts present in the apples, or yeasts that are added specifically for fermentation.
After fermentation is complete, the cider will often be left to mature for several months. At this point, extra sugar is sometimes added to the cider to allow fermentation to continue, and produce a small amount of carbon dioxide to carbonate the cider. However, commercially carbonation is often primarily accomplished via direct injection of carbon dioxide. In the manufacture of some ciders, they may be blended with other, older ciders, to ensure consistency of taste or to alter the flavour.
October 16, 2015
QotD: This explains so much
The entire brain weighs three pounds (1.4 kg) and so is only a small percentage of an adult’s total body weight, typically 2%. But it consumes 20% of all the energy the body uses. Why? The perhaps oversimplified answer is that time is energy.
Neural communication is very rapid — it has to be — reaching speeds of over 300 miles per hour and with neurons communicating with one another hundreds of times per second. The voltage output of a single resting neuron is 70 millivolts, about the same as the line output of an iPod. If you could hook up a neuron to a pair of earbuds, you could actually hear its rhythmic output as a series of clicks.
[…]
Neurochemicals that control communication between neurons are manufactured in the brain itself. These include some relatively well-known ones such as serotonin, dopamine, oxytocin, and epinephrine, as well as acetylcholine, GABA, glutamate, and endocannabinoids. Chemicals are released in very specific locations and they act on specific synapses to change the flow of information in the brain. Manufacturing these chemicals, and dispersing them to regulate and modulate brain activity, requires energy — neurons are living cells with a metabolism, and they get that energy from glucose. No other tissue in the body relies solely on glucose for energy except the testes. (This is why men occasionally experience a battle for resources between their brains and their glands.)
Daniel J. Levitin, The Organized Mind, 2014.
August 23, 2015
The chemistry of ice cream
Compound Interest on the chemical structure of ice cream:
Ice cream is a mainstay of summer – for many, a trip to the beach would be incomplete without one. Despite its seeming simplicity, ice cream is a prime example of some fairly complex chemistry. This graphic takes a look at some of the ingredients that go into ice cream, and the important role they play in creating the finished product. There’s a lot to talk about – whilst the graphic gives an overview, read on for some in-depth ice cream science!
Initially, it might be hard to believe that ice cream could be all that complicated. After all, it’s essentially composed of three basic ingredients: milk, cream, and sugar. How complex can the mixing of three ingredients really be? As it turns out, the answer is: very! Simply mixing the ingredients together, then freezing them, isn’t enough to make a good ice cream. To understand why this is, we’re going to need to talk about each of the component ingredients in turn, and what they bring to the table.
Ice cream is a type of emulsion, a combination of fat and water that usually wouldn’t mix together without separating. However, in an emulsion, the very small droplets of fat are dispersed through the water, avoiding this separation. The manner in which this is accomplished is a result of the chemical properties of molecules in the emulsion.
The fat droplets in ice cream come from the cream used to make it. Fats are largely composed of a class of molecules called triglycerides, with very small amounts (less than 2%) of other molecules such as phospholipids and diglycerides. The triglycerides are made up of a glycerol molecule combined with three fatty acid molecules, as shown in the graphic. The melting temperature of the fats used in ice cream is quite important, as fats that melt at temperatures that are too high give a waxy feel in the mouth, whilst it’s difficult to make stable ice cream with those that melt at too low a temperature. Luckily, dairy fat falls just in the right range! As it happens, you can also make ice cream with palm oil and coconut oil, as their melting temperatures are similar.
August 20, 2015
One of the slickest marketing campaigns of our time
In Forbes, Henry I. Miller and Drew L. Kershen explain why they think organic farming is, as they term it, a “colossal hoax” that promises far more than it can possibly deliver:
Consumers of organic foods are getting both more and less than they bargained for. On both counts, it’s not good.
Many people who pay the huge premium — often more than 100% — for organic foods do so because they’re afraid of pesticides. If that’s their rationale, they misunderstand the nuances of organic agriculture. Although it’s true that synthetic chemical pesticides are generally prohibited, there is a lengthy list of exceptions listed in the Organic Foods Production Act, while most “natural” ones are permitted. However, “organic” pesticides can be toxic. As evolutionary biologist Christie Wilcox explained in a 2012 Scientific American article (“Are lower pesticide residues a good reason to buy organic? Probably not.”): “Organic pesticides pose the same health risks as non-organic ones.”
Another poorly recognized aspect of this issue is that the vast majority of pesticidal substances that we consume are in our diets “naturally” and are present in organic foods as well as non-organic ones. In a classic study, UC Berkeley biochemist Bruce Ames and his colleagues found that “99.99 percent (by weight) of the pesticides in the American diet are chemicals that plants produce to defend themselves.” Moreover, “natural and synthetic chemicals are equally likely to be positive in animal cancer tests.” Thus, consumers who buy organic to avoid pesticide exposure are focusing their attention on just one-hundredth of 1% of the pesticides they consume.
Some consumers think that the USDA National Organic Program (NOP) requires certified organic products to be free of ingredients from “GMOs,” organisms crafted with molecular techniques of genetic engineering. Wrong again. USDA does not require organic products to be GMO-free. (In any case, the methods used to create so-called GMOs are an extension, or refinement, of older techniques for genetic modification that have been used for a century or more.)
August 17, 2015
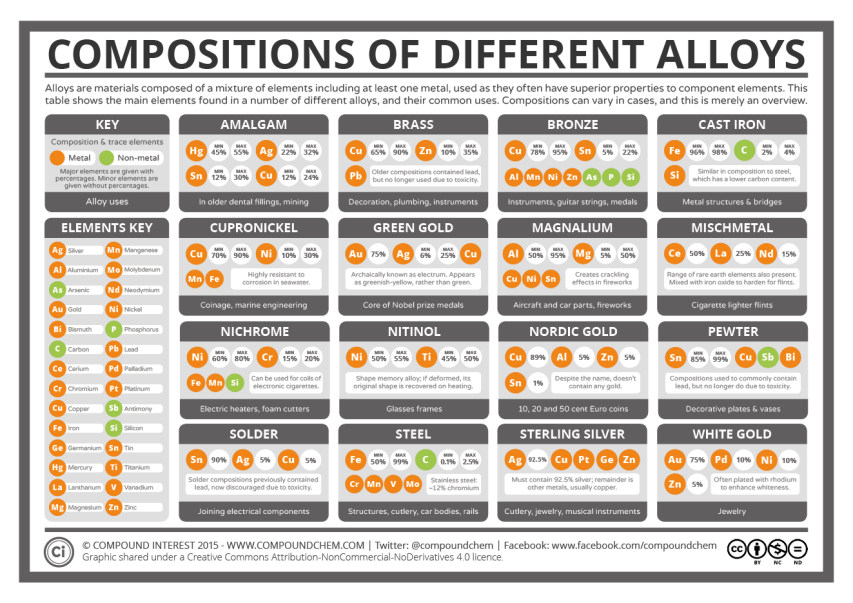
Common metal alloys
Compound Interest looks at the chemical composition of some common metal alloys:
Today’s post looks at an aspect of chemistry we come across every day: alloys. Alloys make up parts of buildings, transport, coins, and plenty of other objects in our daily lives. But what are the different alloys we use made up of, and why do we use them instead of elemental metals? The graphic answers the first of these questions, and in the post we’ll try and answer the second.
First, a little on what alloys are, for anyone unfamiliar with the term. Alloys are a mixture of elements, where at least one of the elements is a metal. There are over 80 metals in the periodic table of elements, and we can mix selections of these different metals in varying proportions, sometimes with non-metals too, to create alloys. Note the use of the word mixture: in the vast majority of cases, alloys are simply intermixed elements, rather than elements that are chemically bonded together.
June 23, 2015
A Genius and A Madman – Fritz Haber I WHO DID WHAT IN WW1?
Published on 22 Jun 2015
Fritz Haber is one of the most famous German scientists. His inventions made it possible to feed an ever growing human population and influence us till this day. But Fritz Haber had a dark side too: His research made the weaponization of gas and the increased production of explosives possible. Find out more about the life of Fritz Haber in our biography.
June 2, 2015
The Chemistry of Cannabis & Synthetic Cannabinoids
Compound Interest posted an infographic on cannabis and synthetic cannabinoids:
In recent years, there’s been an increase in the number of media reports on users of synthetic cannabinoids. Commonly referred to by names such as ‘Spice’ or ‘K2′, the most recent reported case involved five UK students being hospitalised after use. But what are the chemicals present in ‘spice’ and similar drugs, and what are the chemical compounds in cannabis that they aim to mimic? That’s what this graphic and post attempt to answer.
May 30, 2015
The chemistry of gin
Compound Interest looks at the chemical make-up of gin:
For the fifth in the ‘Alcohol Chemistry’ series, we turn to gin. As with other types of alcohol, there are a huge number of different chemical compounds present, but it’s possible to identify a range of significant chemical contributors to its aroma & flavour. Here, we take a look at those compounds and where they come from.
Gin is a spirit that we’ve been making for centuries; although Franciscus Sylvius, a Dutch physician and scientist, is often credited with its discovery in the 17th century, references to gin (or genever as it was also known) exist as far back as the 13th century. Sylvius originally conceived it as an concoction for the treatment of kidney and bladder problems, but its popularity as a recreational drink later soared.
Its popularity in England was spurred by heavy government duties on imported spirits, as well as the fact that gin production was not required to be licensed. This growth in popularity was also accompanied by a gradual decline in its reputation, however, with it being blamed for a range of issues, from social problems such as public drunkenness, to increases in death rates. Gin’s reputation has since largely recovered, although some references to these associations still survive in English parlance – ‘Mother’s Ruin’ is still a widely known alternative name for the spirit.
April 9, 2015
Silent and Deadly – GAS WARFARE IN WORLD WAR 1
Published on 7 Apr 2015
All soldiers feared poison gas but all sides developed deadlier and more perfidious kinds of chemical agents. Indy tells you everything about gas warfare in World War 1 in this special episode.
April 3, 2015
Updating the old saying “where there’s muck, there’s money”
According to this story in the Guardian, a typical city of one million people poops out $13 million in (potentially recoverable) precious metals every year:
Sewage sludge contains traces of gold, silver and platinum at levels that would be seen as commercially viable by traditional prospectors. “The gold we found was at the level of a minimal mineral deposit,” said Kathleen Smith, of the US Geological Survey.
Smith and her colleagues argue that extracting metals from waste could also help limit the release of harmful metals, such as lead, into the environment in fertilisers and reduce the amount of toxic sewage that has to be buried or burnt.
“If you can get rid of some of the nuisance metals that currently limit how much of these biosolids we can use on fields and forests, and at the same time recover valuable metals and other elements, that’s a win-win,” she said.
A previous study, by Arizona State University, estimated that a city of 1 million inhabitants flushed about $13m (£8.7m) worth of precious metals down toilets and sewer drains each year.
The task of sifting sewage for microscopic quantities of gold may sound grim, but it could have a variety of unexpected benefits over traditional gold mining. The use of powerful chemicals, called leachates, used by the industry to pull metals out of rock is controversial, because these chemicals can be devastating to ecosystems when they leak into the environment. In the controlled setting of a sewage plant, the chemicals could be used liberally without the ecological risks.
February 9, 2015
Paradoxically, rising cancer deaths are a form of good news
Last month, in his Times column, Matt Ridley explained why — until we discover a treatment for aging itself — rising cancer rates are a weird form of good news:
If we could prevent or cure all cancer, what would we die of? The new year has begun with a war of words over whether cancer is mostly bad luck, as suggested by a new study from Johns Hopkins School of Medicine, and over whether it’s a good way to die, compared with the alternatives, as suggested by Dr Richard Smith, a former editor of the BMJ.
It is certainly bad luck to be British and get cancer, relatively speaking. As The Sunday Times reported yesterday, survival rates after cancer diagnosis are lower here than in most developed and some developing countries, reflecting the National Health Service’s chronic problems with rationing treatment by delay. In Japan, survival rates for lung and liver cancer are three times higher than here.
Cancer is now the leading cause of death in Britain even though it is ever more survivable, with roughly half of people who contract it living long enough to die of something else. But what else? Often another cancer.
In the western world we’ve conquered most of the causes of premature death that used to kill our ancestors. War, smallpox, homicide, measles, scurvy, pneumonia, gangrene, tuberculosis, stroke, typhoid, heart disease and cholera are all much rarer, strike much later in life or are more survivable than they were fifty or a hundred years ago.
The mortality rate in men from coronary heart disease, for instance, has fallen by an amazing 80 per cent since 1968 — for all age groups. Mortality rates from stroke in both sexes have halved in 20 years. Cancer’s growing dominance of the mortality tables is not because it’s getting worse but because we are avoiding other causes of death and living longer.
It is worth remembering that some scientists and anti-pesticide campaigners in the 1960s were convinced that by now lifespans would be much shorter because of cancer caused by pesticides and other chemicals in the environment.
In the 1950s Wilhelm Hueper — a director of the US National Cancer Institute and mentor to Rachel Carson, the environmentalist author of Silent Spring — was so concerned that pesticides were causing cancer that he thought the theory that lung cancer was caused by smoking was a plot by the chemical industry to divert attention from its own culpability: “Cigarette smoking is not a major factor in the causation of lung cancer,” he insisted.
In fact it turns out that pollution causes very little cancer and cigarettes cause a lot. But aside from smoking, most cancers are indeed bad luck. The Johns Hopkins researchers found that tissues that replicate their stem cells most run the highest risk of cancer: basal skin cells do ten trillion cell divisions in a lifetime and have a million times more cancer risk than pelvic bone cells which do about a million cell divisions. Random DNA copying mistakes during cell division are “the major contributors to cancer overall, often more important than either hereditary or external environmental factors”, say the US researchers.
(Emphasis mine.)
To sum it up, until or unless medical research finds a way to stop the bodily effects of aging, cancer becomes the most likely way for all of us to die. Cancer is a generic rather than a specific term — it’s what we use to describe the inevitable breakdown of the cellular division process that happens millions or even trillions of times over our lifetime. As Ridley puts it, “even if everybody lived in the healthiest possible way, we would still get a lot of cancer.” I’m not a scientist and I don’t even play one on TV, but I suspect that the solution to cancers of all kinds are to boost our immune systems to more quickly identify aberrant cells in our bodies before they start reproducing beyond the capability of the immune system to handle. The short- to medium-term solution to cancer may be to make us all a little bit cyborg…
January 26, 2015
Balancing the art and the science in winemaking
In Cosmos, Andrew Masterson investigates what is still an art and what has been codified as science:
“With commercial yeast you get certainty – you can sleep at night,” says Bicknell. “But how do you make wine more interesting? You exploit the metabolic processes of different yeast species.”
Bicknell’s faith in wild yeasts adds stress at fermentation time, but the pay-off is multi-award-winning wines regularly acknowledged as some of the best in Australia. “The wines do taste different, even if there’s no way you can show that statistically,” Bicknell says. “The only way to really know is to taste.”
Exploiting the diverse and fluctuating populations of wild yeasts found on the plants, fruit and in the air of vineyards is “the new black” (not to mention red and white) in oenology. The practice is becoming more commonplace among artisan winemakers. Even some of the giant commercial wine corporations are investing in the method.
Wild fermentation, says Bicknell, represents the intersection of science, craft and philosophy. But it could also form the basis of a profound shift in the narrative of wine. The more we study winemaking’s microbes, the more it appears they might explain one of the wine industry’s most beloved, but vaguest, terms: terroir.
“Terroir is a wonderful marketing term,” says David Mills, a microbiologist at UC Davis, who studies microbes in wine. “But it’s not a science.”
The French word terroir is difficult to translate. The closest translation is “soil”, but that is just one of its components. Terroir connotes the unique sense of place – the soils, the topography and the microclimate. It’s what makes the wines of Bordeaux or Australia’s Coonawarra so distinctive, and so inimitable.
Sommeliers like Ren Lim, former captain of the Oxford University Blind Tasting Society (and a PhD biophysics student) will tell you merely from swirling a mouthful of Cabernet Sauvignon which Australian winery produced it.
“The ones from Margaret River often give off a more pronounced green pepper note, a note found commonly in Cabernets grown in regions which experience pronounced maritime influences. Coonawarra Cabernets are somewhat different and unique in their own way. They are often minty and have a eucalyptus or menthol note in addition to the usual ripe blackcurrant notes. The green pepper note is often suppressed under the menthol notes. Nonetheless, the Cabernet structure remains in both these wines.”
It’s a feat that Mills does not question. “I don’t doubt regionality exists, but what causes it is a whole other set of issues.”
January 6, 2015
The amazing – and scary – power of testosterone
A throw-away comment on the experiences of female-to-male transgender people by Scott Alexander:
… I could hunt down all of the stories of trans men who start taking testosterone, switch to a more male sex drive, and are suddenly like “OH MY GOD I SUDDENLY REALIZE WHAT MALE HORNINESS IS LIKE I THOUGHT I KNEW SEXUAL FRUSTRATION BEFORE BUT I REALLY REALLY DIDN’T HOW DO YOU PEOPLE LIVE WITH THIS?”
The author of the last link has this to say about the impact of testosterone on his life:
One of the most interesting things about the effects of testosterone and trans men is that we have something else to compare it to. Non-trans men do not. And non-trans women do not, which is why I wrote the post “It’s the Testosterone: What Straight Women Should Know.”
When I started testosterone a dozen years ago, I expected my sex drive to increase. The “horror” stories are a part of trans man lore, passed down from generation to generation as we all gear up for male adolescence, no matter how old we are, and take out a line of credit at the adult toy store.
And it did increase, within about four days of my first shot, and I basically squirmed a lot for two years before I got used to it. But I was planning for that. Here are the things that took me by surprise:
> It became very focused on one thing – the goal, the prize, the end. That doesn’t mean that I was not able to “make love.” What it does mean is that there was a madness to my method, because it was goal-oriented. There was a light at the end of the tunnel. There was a pot of gold at the end of the rainbow. There was an unguarded hoop just waiting for a slam dunk – score!
> It became very visual. I saw it, I wanted it – whatever it was. This was a new experience for me, because, in the past, I had not been aroused so much by pictures and body parts (or pictures of body parts) as I had been by words – erotic descriptions, stories, and things said to me.
> It became very visceral – instinctual – with a need to take care of it. It had very little to do with romance or even an attraction that made sense intellectually. You’re hungry, you eat. There was a matter-of-factness about it, especially when I was by myself. Hmm … peanut butter sandwich sounds good. Okay, done. Let’s move on.
And from the linked post:
Whenever I speak at a college class (which I did this week), I inevitably get the question about testosterone and sex drive (because college kids are still young enough to be thinking about sex most of the time).
And I tell them the truth, which is that, at least for me and most guys I know, testosterone sends your sex drive straight through the roof and beyond the stratosphere. NASA should honestly use it for fuel to get those rockets (which are really just larger-than-life phallic symbols) to the moon. It is a very powerful aphrodisiac, and way better than oysters, which tend to be slimy.
Testosterone not only increased my sex drive ten-fold, but changed the nature of it as well. It became less diffuse and more goal-oriented, which is probably how the word “score” entered the sexual lexicon. It also, in certain situations, became less about any other person and more about me.