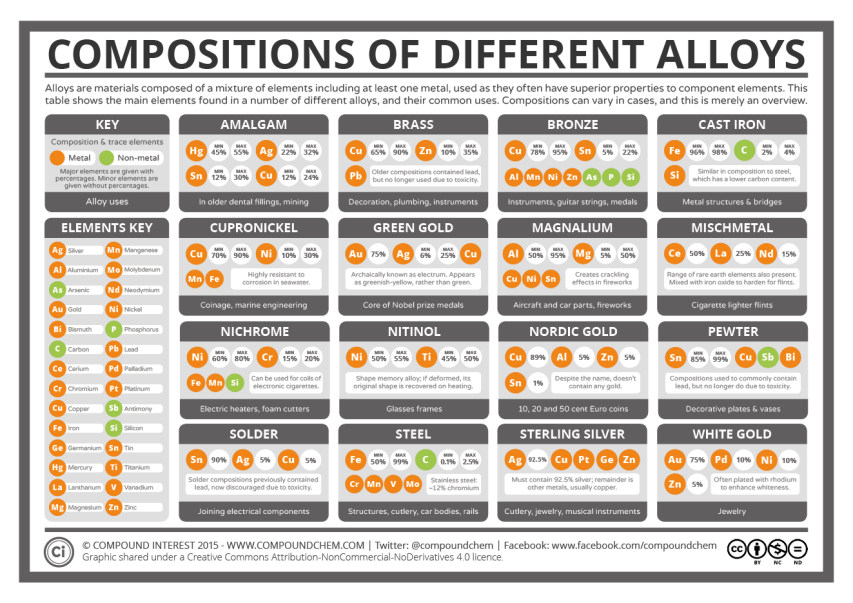
Compound Interest looks at the chemical composition of some common metal alloys:
Today’s post looks at an aspect of chemistry we come across every day: alloys. Alloys make up parts of buildings, transport, coins, and plenty of other objects in our daily lives. But what are the different alloys we use made up of, and why do we use them instead of elemental metals? The graphic answers the first of these questions, and in the post we’ll try and answer the second.
First, a little on what alloys are, for anyone unfamiliar with the term. Alloys are a mixture of elements, where at least one of the elements is a metal. There are over 80 metals in the periodic table of elements, and we can mix selections of these different metals in varying proportions, sometimes with non-metals too, to create alloys. Note the use of the word mixture: in the vast majority of cases, alloys are simply intermixed elements, rather than elements that are chemically bonded together.